-
Studying the mechanisms of the human mind.
With the aim of understanding the neural mechanisms of our minds and their individual differences and pathologies, we conduct research on a wide range of issues and use functional neuroimaging techniques such as an fMRI.”

What is the “mind” of a human being? If the mind is in the brain, how can we know? The mind and brain evolve, or develop, by adapting to a complex environment. The human brain is what created society, culture, the economy, and, needless to say, it has also greatly changed the environment in which we live. Nevertheless, the ontogeny of our brain still recapitulates its phylogeny and maintains a close connection between the brain and body. How do our mind and brain modulate the interactions between our bodies and the world? Why do we see individual difference and why do things sometimes not work the way we expect? In the future, how will the human brain evolve and change the makeup of our minds and the world?
The brain gains and integrates input information from the external and internal environment through sensory devices, recognizes situations by comparing them against our memories, and outputs adaptive behavior as a motor action. In the same way, information processing is achieved by a combination of many mental elements (functional modules). We are investigating these mental elements and combinations of them using functional neuroimaging techniques, such as functional MRI (fMRI), together with varied psychological measurements. Our research target spans a wide range of subject matter: from human-specific higher mental functioning, such as the comprehension and memory of complex situations in the real world, to empathy and making inferences about another person’s mind, to behavioral decision making based on the previous two processes, to basic physiological information processing critical for survival as an animal.
Figure Captions:
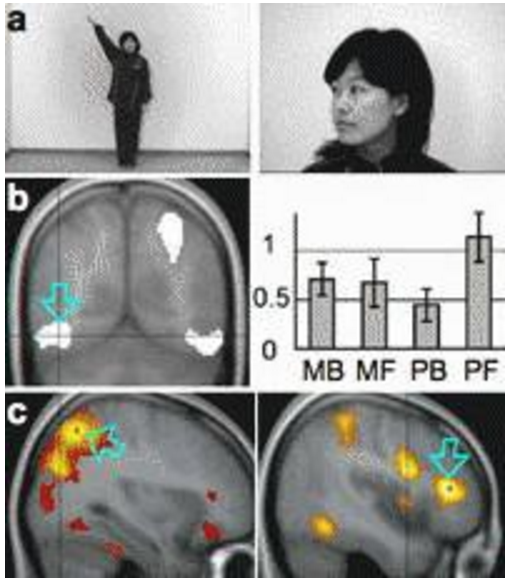
The ability to detect eye-gaze movement is observed in infants and is hypothesized to play a critical role in the development of the ability to infer another person’s mental state, known as “Theory of mind”. Our fMRI experiment using visual stimuli that shows eye-gaze movement (a) and a pictorial story comprehension task (b) revealed a neural response in the posterior part of the right middle temporal gyrus (blue arrowhead) to the eye-gaze movement covariate as well as responses at the temporal pole (green arrowhead) and in the ventromedial prefrontal cortex (purple arrowhead) during story comprehension (c), thus supporting the hypothesis (Sugiura et al., Soc Cogn Affect Neurosci, 2013, doi:10.1093/scan/nst119).
Examples:
[ms_accordion style=”simple” type=”1″ class=”” id=””] [ms_accordion_item title=”Face-specific and domain-general characteristics of cortical responses during self-recognition.” color=”#333333″ background_color=”” close_icon=”” open_icon=”” status=”close” role=”tab”]The ability of visual self-recognition in animals and infants is considered a hallmark of the domain-general cognitive representation of the self, which also underpins higher social ability. Cortical regions activated during self-face recognition in human adults have been accordingly expected to play the domain-general role in self-processing. However, there is no evidence of the involvement of this network in non-face domains.
We compared cortical responses during face and name recognition (Fig. 1) of self, a friend, and an unfamiliar person, using functional magnetic resonance imaging (fMRI) [1]. Recognition of the self-face activated the right inferior frontal, precentral, supramarginal, and bilateral ventral occipitotemporal regions (Fig. 2 red), consistent with previous findings [2,3], whereas these regions did not show self-specific activation during name recognition. During both face and name recognitions, increased activation for the friend and unfamiliar person than for the self was observed in the bilateral temporoparietal regions (Fig. 2 blue). These results suggest that the role of the self-specific networks during face recognition is not domain-general, but rather face-specific. Instead, the reduced temporoparietal activation is a domain-general characteristic of the cortical response during self-recognition, which may reflect suppression of an automatic preparatory process for social interaction [4], possibly paralleling the disappearance of social behavior to the mirrored self-image at the emergence of self-recognition in animals and infants.
Fig.1 Examples of stimuli: face (left) and name (right).
Fig.2 Face-specific activation (red) and domain-general inactivation (blue) during self-recognition. Left and right lateral views (left and right panels, respectively).
Reference
1. Sugiura M, Sassa Y, Jeong H, Horie K, Sato S, Kawashima R.
“Face-specific and domain-general characteristics of cortical responses during self-recognition.”
Neuroimage. 2008;42:414-22.2. Sugiura M, Watanabe J, Maeda Y, Matsue Y, Fukuda H, Kawashima R.
“Cortical mechanisms of visual self-recognition.”
Neuroimage. 2005;24:143-9.3. Sugiura M, Sassa Y, Jeong H, Miura N, Akitsuki Y, Horie K, Sato S, Kawashima R.
“Multiple brain networks for visual self-recognition with different sensitivity for motion and body part.”
Neuroimage. 2006;32:1905-17.4. Sassa Y, Sugiura M, Jeong H, Horie K, Sato S, Kawashima R.
“Cortical mechanism of communicative speech production.”
Neuroimage. 2007;37:985-92.
[/ms_accordion_item] [ms_accordion_item title=”Cortical mechanisms of communicative speech production.” color=”#333333″ background_color=”” close_icon=”” open_icon=”” status=”close” role=”tab”]Speech in everyday verbal communication is a social behavior using language and requires conformity not only to linguistic rules but also to behavior that is appropriate for social interaction. However, little is known about the brain mechanism for the behavior aspects of communicative speech production.
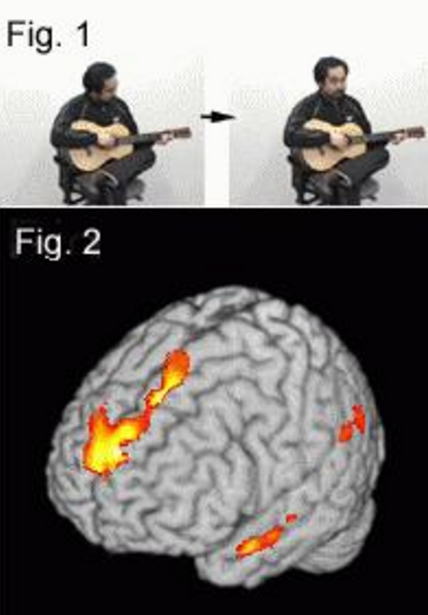
We performed a functional magnetic resonance imaging (fMRI) study to investigate the cortical activation while normal subjects casually talked to an actor (communication task) or verbally described a situation (description task) while observing video clips of an action performed by an actor in a typical daily situation (Fig. 1) [1].
Significantly higher activation was observed during the communication task than during the description task in the medial prefrontal cortex (polar and dorsal parts), the bilateral anterior superior temporal sulci, and the left temporoparietal junction (Fig. 2). The results suggest that these regions play a role in the behavioral aspects of communicative speech production. Interestingly, all of these areas coincide with areas that have been implicated in the theory of mind, or mentalization, suggesting that a role for these regions in understanding the context of social interaction, which is essential for communication.
Fig.1 Example of stimulus. (adapted by Sassa et al., 2007)
Fig.2 Selective activation for communicative speech production.
Reference
1. Sassa Y, Sugiura M, Jeong H, Horie K, Sato S, Kawashima R
“Cortical mechanism of communicative speech production.”
Neuroimage. 37: 985-992, 2007.
[/ms_accordion_item] [ms_accordion_item title=”Cortical Mechanisms Underlying Comprehension of Implicit Meanings in Social Situations.” color=”#333333″ background_color=”” close_icon=”” open_icon=”” status=”close” role=”tab”]To understand implicit social meanings, the interaction of literal meanings and relevant information in a situational context is important.
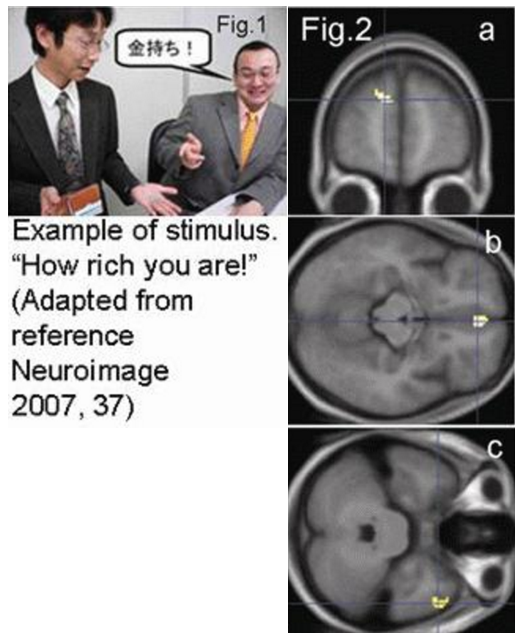
Using fMRI, we investigated cortical mechanisms underlying the processing of implicit meanings, particularly irony, in realistic social situations, focusing on contextual interactions [1]. Healthy subjects were shown pictures depicting daily communicative situations during judgment tasks involving situational appropriateness and literal correctness (Fig.1). The left medial prefrontal cortex showed significantly greater activation during tasks involving situational judgments than during literal judgments (Fig.2a). The right temporal pole was activated task-independently during irony-specific processing (Fig.2b). The medial orbitofrontal cortex was activated task-dependently during irony processing in situational judgment tasks (Fig.2c). These regions have been reported to be involved in theory of mind, and have not been implicated in previous studies on the linguistic processing of implicit meanings. This suggests that the intentional assessment of situational appropriateness for task execution is carried out in the left medial prefrontal cortex, whereas irony is processed in the right temporal pole by assessing situational context automatically, and is judged based on the situational context in the medial orbitofrontal cortex. Our results show that the processing of implicit meanings and irony in contextually rich situations depends on brain mechanisms involved in the “theory of mind,” based on processing relevant information in a situational context, and suggest different functions in each region.

Reference
1. Wakusawa K, Sugiura M, Sassa Y, Jeong H, Horie K, Sato S, Yokoyama H, Tsuchiya S, Inuma K, Kawashima R.
“Comprehension of Implicit Meanings in Social Situations Involving Irony: A Functional MRI Study.”
Neuroimage 2007; 37: 1417-1426.
[/ms_accordion_item] [ms_accordion_item title=”Multiple brain networks for visual self-recognition with different sensitivities for motion and body parts.” color=”#333333″ background_color=”” close_icon=”” open_icon=”” status=”close” role=”tab”]Multiple brain networks may support visual self-recognition. It has been hypothesized that the left ventral occipito-temporal cortex processes one’s own face as a symbol, and the right parieto-frontal network processes self-image in association with motion-action contingency[1].
Using functional magnetic resonance imaging, we first tested these hypotheses based on the prediction that these networks preferentially respond to a static self-face and to moving one’s whole body, respectively [2]. Brain activation specifically related to self-image during familiarity judgment was compared across four stimulus conditions comprising a two factorial design: factor Motion contrasted movie (M) and picture (P), and factor Body part a whole body (B) and face (F) (Fig. 1a). Second, we attempted to segregate self-specific networks using a principal component analysis (PCA) [3], assuming an independent pattern of inter-subject variability in activation over the four stimulus conditions in each network. The bilateral ventral occipito-temporal and the right parietal and frontal cortices exhibited self-specific activation. The left ventral occipito-temporal cortex exhibited greater self-specific activation for PF than for PB (Fig. 1b), consistent with the prediction for this region. The activation profiles of the right parietal and frontal cortices did not show preference for MB predicted by the assumed roles of these regions. The PCA extracted two cortical networks, one with its peaks in the right posterior, and another in frontal cortices (Fig. 1c). The results thus supported and provided evidence of multiple brain networks for visual self-recognition.
Fig.1 Examples of stimuli. b Self-specific activation in the left ventral occipito-temporal cortex, and its activation profile. c Two independent self-specific cortical networks extracted by PCA. (adapted from reference 2).
Reference
1. Sugiura M, Watanabe J, Maeda Y, Matsue Y, Fukuda H, Kawashima R.
“Cortical mechanisms of visual self-recognition.”
Neuroimage. 2005;24:143-9.2. Sugiura M, Sassa Y, Jeong H, Miura N, Akitsuki Y, Horie K, Sato S, Kawashima R.
“Multiple brain networks for visual self-recognition with different sensitivity for motion and body part.”
Neuroimage. 2006;32:1905-17.3. Sugiura M, Friston KJ, Willmes K, Shah NJ, Zilles K, Fink GR.
“Analysis of intersubject variability in activation: An application to the incidental episodic retrieval during recognition test.”
Hum Brain Map. 2007;28:49-58.
[/ms_accordion_item] [ms_accordion_item title=”Cortical mechanisms of person representation: recognition of famous and personally familiar names.” color=”#333333″ background_color=”” close_icon=”” open_icon=”” status=”close” role=”tab”]Personally familiar people are likely to be represented more richly in episodic, emotional, and behavioral contexts than famous people, who are usually represented predominantly in semantic context.
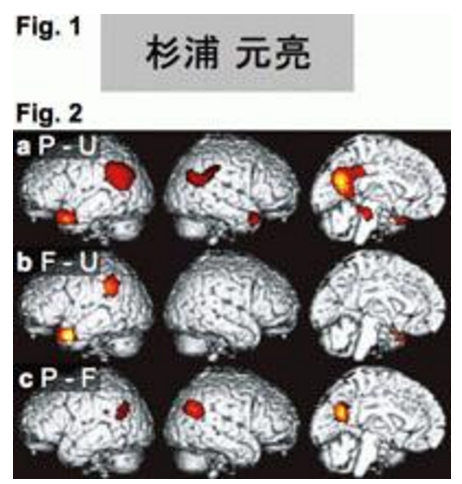
To reveal cortical mechanisms supporting this differential person representation, we compared cortical activation during name recognition tasks between personally familiar and famous names, using an event-related functional magnetic resonance imaging (fMRI) [1]. Normal subjects performed familiar- or unfamiliar-name detection tasks during visual presentation of personally familiar (P), famous (F), and unfamiliar (U) names. The bilateral temporal poles and anterolateral temporal cortices, as well as the left temporoparietal junction, were activated in the contrasts P-U and F-U to a similar extent. The bilateral occipitotemporoparietal junctions, precuneus, and posterior cingulate cortex showed activation in the contrasts P-U and P-F. Together with previous findings, differential activation in the occipitotemporoparietal junction, precuneus, and posterior cingulate cortex between personally familiar and famous names is considered to reflect differential person representation. The similar extent of activation for personally familiar and famous names in the temporal pole and anterolateral temporal cortex is consistent with the associative role of the anterior temporal cortex in person identification, which has been conceptualized as a person identity node in many models of person identification. The left temporoparietal junction was considered to process familiar written names. The results illustrated the neural correlates of the person representation as a network of discrete regions in the bilateral posterior cortices, with the anterior temporal cortices having a unique associative role.
Fig.1 Example of visual stimuli (Family and first names in Kanji characters).
Fig.2 Statistically significant differential cortical activation during name recognition. P: personally familiar name, F: famous name, and U: unfamiliar name.
Reference
1. Sugiura M, Sassa Y, Watanabe J, Akitsuki Y, Maeda Y, Matsue Y, Fukuda H, Kawashima R.
“Cortical mechanisms of person representation: recognition of famous and personally familiar names.”
Neuroimage. 2006;31:853-60.
[/ms_accordion_item] [ms_accordion_item title=”Cortical mechanisms of visual self-recognition.” color=”#333333″ background_color=”” close_icon=”” open_icon=”” status=”close” role=”tab”]Several lines of evidence have suggested that visual self-recognition is supported by a special brain mechanism; however, its functional anatomy is of great controversy.
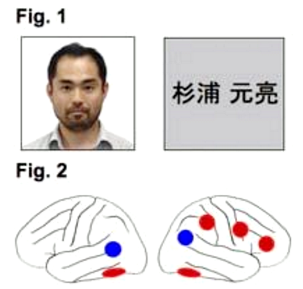
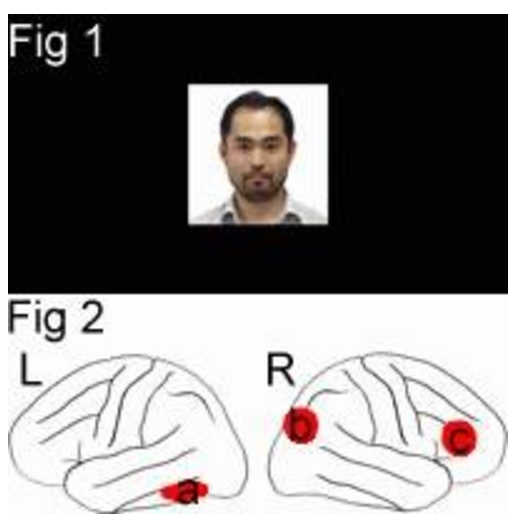
We performed an event-related functional magnetic resonance imaging (fMRI) study to identify brain regions selectively involved in recognition of one’s own face [1]. We presented pictures of each subject’s own face (SELF) and a prelearned face of an unfamiliar person (CONT), as well as two personally familiar faces with high and low familiarity (HIGH and LOW, respectively) to test selectivity of activation to the SELF face (Fig. 1). Compared with the CONT face, activation selective to the SELF face was observed in the left fusiform gyrus (Fig. 2a), the right occipito-temporo-parietal junction (Fig. 2b) and frontal operculum (Fig. 2c). On the contrary, the temporoparietal junction in both the hemispheres and the left anterior temporal cortex, which were activated during recognition of HIGH and/or LOW faces, were not activated during recognition of the SELF face. The results confirmed the partial distinction of the brain mechanism involved in recognition of personally familiar faces and that in recognition of one’s own face.
Fig.1 Examples of stimulus.
Fig.2 Selective activation for one’s own face. L: left hemisphere, R: right hemisphere.
Reference
1. Sugiura M, Watanabe J, Maeda Y, Matsue Y, Fukuda H, Kawashima R.
“Cortical mechanisms of visual self-recognition. Neuroimage.”
2005;24:143-9.
[/ms_accordion_item] [ms_accordion_item title=”Mental visual synthesis is originated in the fronto-temporal network of the left hemisphere.” color=”#333333″ background_color=”” close_icon=”” open_icon=”” status=”close” role=”tab”]Mental visual synthesis is the capacity for experiencing, constructing, or manipulating ‘mental imagery’. To our knowledge no brain imaging study has been performed that directly investigated the relationship between brain activity and mental visual synthesis that is the basis of imagination.
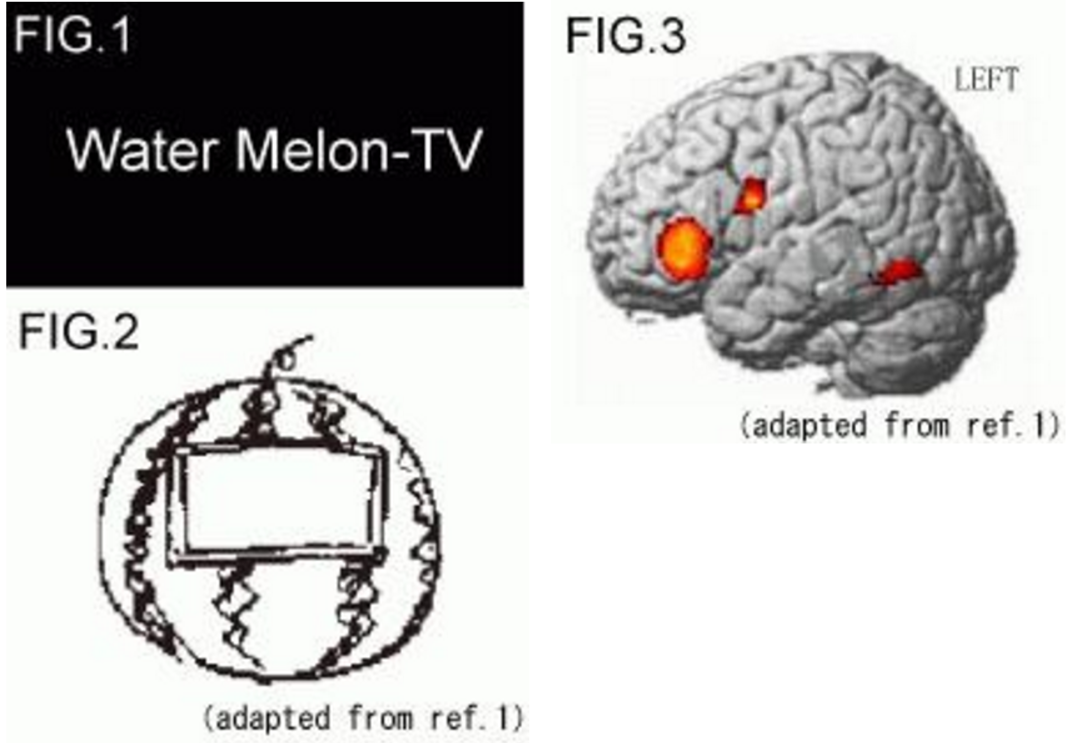
To investigate brain networks involved in mental visual synthesis, brain activity was measured in right-handed healthy volunteers during mental imagery tasks, in which the subjects were instructed to imagine a novel object, that does not exist in the real world, by composing it from two visually presented words associated with a real object or two achromatic line drawings of a real object(Fig.1), using functional magnetic resonance imaging (fMRI). Both tasks activated the same areas in the inferior frontal and inferior temporal cortices of the left hemisphere(Fig.2). Our results indicate that the source of mental visual synthesis may be formed by activity of a brain network consisting of these areas, which are also involved in semantic operations and visual imagery.
Fig.1 Examples of stimuli.
Fig.2 A line drawing subject drew after an experiment ( Water Melon-TV).
Fig.2 Selective activation for “Mental Visual synthesis”.
Reference
1. Yomogida Y, Sugiura M, Watanabe J, Akitsuki Y, Sassa Y, Sato T, Matsue Y, Kawashima R.
“Mental visual synthesis is originated in the fronto-temporal network of the left hemisphere.”
CEREBRAL CORTEX, 2004;14: 1376-1383
[/ms_accordion_item] [ms_accordion_item title=”Context-dependent cortical activation in response to financial rewards and penalties.” color=”#333333″ background_color=”” close_icon=”” open_icon=”” status=”close” role=”tab”]Receiving rewards or penalties is not a simply passive behavior. Animals should predict potential rewards/penalties, distinguish their nature, and respond appropriately by changing their attentional or arousal level for successful adaptive behavior.
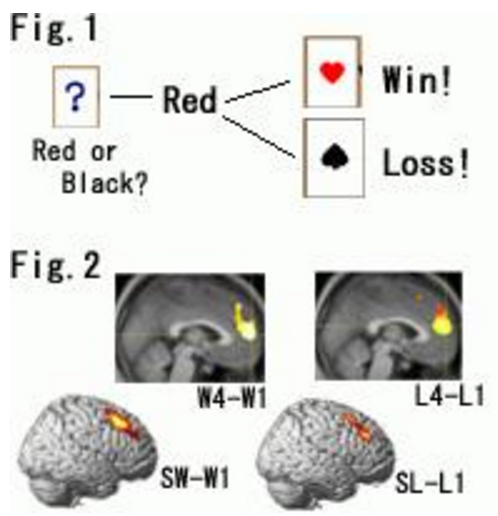
A prior emotional experience with streaks of rewards/penalties appears to shape the subjective “feeling” of an incoming expected reward/penalty or an unexpected one for that matter. Moreover, there seems to be dissociable neural responses that are dependent on the psychological context in which abstract rewards and penalties are received in the human brain. An event-related fMRI technique was used to assess neural responses to financial reward and penalty during a simple gambling task. We attempted to determine whether brain activities are dependent on the unique context of an event sequence. Thirty-six healthy volunteers participated in the study. The task was to guess the color of the suit of a card on each trial and to respond by pressing a button. Every correct response (“win”) and incorrect response (“loss”) was associated with financial reward and penalty, respectively (Fig.1). The magnitude of reward or penalty in each trial did not change; however, the subjects’ self-reported emotional arousal was significantly higher for the events of “the fourth win of four wins in a row (W4)” and “the fourth loss of four losses in a row (L4)”. We also found that the bilateral anterior cingulate and medial prefrontal cortices were specifically activated when the subjects experienced “the fourth win of four wins in a row (W4)” and “the fourth loss of four losses in a row (L4)”(Fig.2: upper panel). When the subjects experienced “a win following four losses in a row (SW)” or “a loss following four wins in a row (SL)”, the right dorsolateral prefrontal cortex was specifically activated (Fig.2: lower panel). Our data indicate that there exist brain activities associated with the event-sequence context in which abstract reward or penalty is received. These context-dependent activities appear to be crucial for adapting oneself to new circumstances and may account for clinical symptoms of various mental illnesses in which dysfunction of these regions has been reported.
Fig.1 Example of gamble task.
Fig.2 Depending on the nature of the unique context (i.e., repetition of win or loss vs. switching from a winning or losing streak to the opposite event), specific brain regions were activated. W4: the fourth win of four wins in a row.
L4: the fourth loss of four losses in a row
SW: a win following four losses in a row
SL: a loss following four wins in a row
W1/L1: control event
Reference
1. Akitsuki Y, Sugiura M, Watanabe J, Yamashita K, Sassa Y, Awata S, Matsuoka H, Matsue Y, Fukuda H, Kawashima R.
“Context-dependent cortical activation in response to financial reward and penalty: An event-related fMRI study.”
Neuroimage 19: 1674-1685, 2003
[/ms_accordion_item] [/ms_accordion] -
For a smart aging society.
We are working to develop a system for the improvement of brain functions in the elderly. Our system will be capable of preventing and improving senile dementia, and it will also support healthy brain development in children.

This study aims to find ways of retaining and improving human brain functions, cognitive functions, emotional states, psychiatric and neurological diseases and disorders through external stimuli or mental work designed using our brain science knowledge and techniques. Ultimately, we hope to see the creation of a sustainable society in which people can live full, healthy, and spiritually affluent lives.
As people age, cognitive activities that require knowledge and wisdom improve, but the function of the prefrontal region, which is only well-developed in humans, declines steadily beginning right after completion of its growth. Presuming that what is lost in aging is, for the most part, the result of decreased functioning of the prefrontal region, we have developed a system primarily to retain and improve the functioning of the prefrontal region in healthy people. The system (learning therapy) we have proposed to improve and prevent symptoms of dementia has widely been put into practice in society.
This study encompasses a broad range of research about humans. Topics include (1) fundamental research of brain science with functional brain imaging; (2) life intervention experiments targeting normal subjects and subjects with psychiatric and neurological disorders (the study’s outcome measures are psychological tests and MRI scans); (3) practical social studies; and (4) social action programs.
Examples:
[ms_icon icon=”fa-check” size=”16″ color=”#333333″ icon_box=”no” class=”” id=””] Mobility and Smart Aging Project
Combining the “seeds” of Tohoku University, we are developing a completely new type of motorized vehicle with added value, which offsets the negative aspects of traditional cars (decline in physical strength, environmental impact, etc.) and improves the mental and physical health of the driver just by being driven.
[ms_icon icon=”fa-check” size=”16″ color=”#333333″ icon_box=”no” class=”” id=””] Development of working memory training method
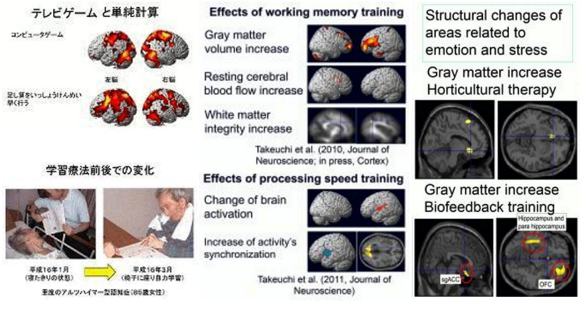
Using the result of our functional brain imaging studies, we are developing tasks for healthy people which place a burden on their working memory. By putting the tasks into practice as life interventions, we are improving working memory. Additionally, we are examining the interventions’ generalized effects on other cognitive functions and on various neural mechanisms, such as brain structures, brain functions, brain perfusion and functional connectivity. Besides working memory training, we have investigated similar psychological and neuroimaging interventional studies of simple processing speed training, fast and simple neural calculation training, and multitasking training.
[ms_icon icon=”fa-check” size=”16″ color=”#333333″ icon_box=”no” class=”” id=””] Interventional studies to improve mental health
We have performed a number of intervention studies to improve the mental health of adult subjects and disaster victims. These interventions include horticultural therapy (growing plants) and biofeedback training, which involves obtaining biosignals such as cerebral blood flow and heart rate from machines and learning to control these signals. We have investigated whether these training activities improve or alter mental health, stress hormones, and brain structures.

Reference:
1. Takeuchi H, Taki Y, Nouchi R, Hashizume H, Sekiguchi A, Kotozaki Y, Nakagawa S, Miyauchi CM, Sassa Y, Kawashima R.
“Effects of working memory-training on functional connectivity and cerebral blood flow during rest.”
Cortex, in press.2. Takeuchi H, Kawashima R.
“Effects of processing speed training on cognitive functions and neural systems. (review)”
Reviews in the Neurosciences, 23(3): 289-301, 2012.3. Takeuchi H, Taki Y, Sassa Y, Hashizume H, Sekiguchi A, Fukushima A, Kawashima R.
“Working memory training using mental calculation impacts regional gray matter of the frontal and parietal regions.”
PLoS ONE, 6(8): e23175:1-12, 2011.4. Takeuchi H, Taki Y, Hashizume H, Sassa Y, Nagase T, Nouchi R, Kawashima R.
“Effects of training of processing speed on neural systems.”
Journal of Neuroscience, 31(34): 12139-12148, 2011.5. Takeuchi H, Taki Y, Kawashima R.
“Effects of working memory training on cognitive functions and neural systems. (review)”
Reviews in the Neurosciences, 21(6): 427-449, 2010.6. Takeuchi H, Sekiguchi A, Taki Y, Yokoyama S, Yomogida Y, Nozomi K, Yamanouchi T, Suzuki S, Kawashima R.
“Training of working memory impacts structural connectivity.”
Journal of Neuroscience, 30(9): 3297-303, 2010. Scored in Faculty of 10007. Uchida S, Kawashima R.
“Reading and solving arithmetic problems improve cognitive functions of normal aged people –A randomized controlled study.”
Age. 30: 21-29, 2008.8. Kawashima R, Okita K, Yamazaki R, Tajima N, Yoshida H, Taira M, Iwata K, Sasaki T, Maeyama K, Usui N, Sugimoto K.
“Reading aloud and arithmetic calculation improve frontal function of people with dementia.”
Journals of Gerontology, Series A, Biological Sciences and Medical Sciences, 60A: 380-384, 2005. -
We are conducting two types of research currently. The first is basic and applied social research on neural decoding with the aim of reading individual cognitive states from brain activity, and the second is research which connects communication and collective neural synchrony using simultaneous multi-brain recordings.

It is difficult to be aware of how our own cognitive process is working or to guess another person’s mental state just by looking at them. Yet recent progress in neural function measurement technologies is on the way to enabling continuous brain activity measurement in everyday life. Decoding cognitive and emotional information from brain activity will open a wide range of potential applications.
In our move forward to apply brain science to the real world, we are conducting basic research and making technical developments that advance the neural decoding of individual mental states, such as attention, motivation, preference, and mood, making full use of the newest functional measurement techniques (NIRS, fMRI, EEG, MEG). These studies also extend to such social applications as the improvement of people’s cognition and mental health, assistance in intellectual activities, enhancement of safety, and design support for products and marketing campaigns.
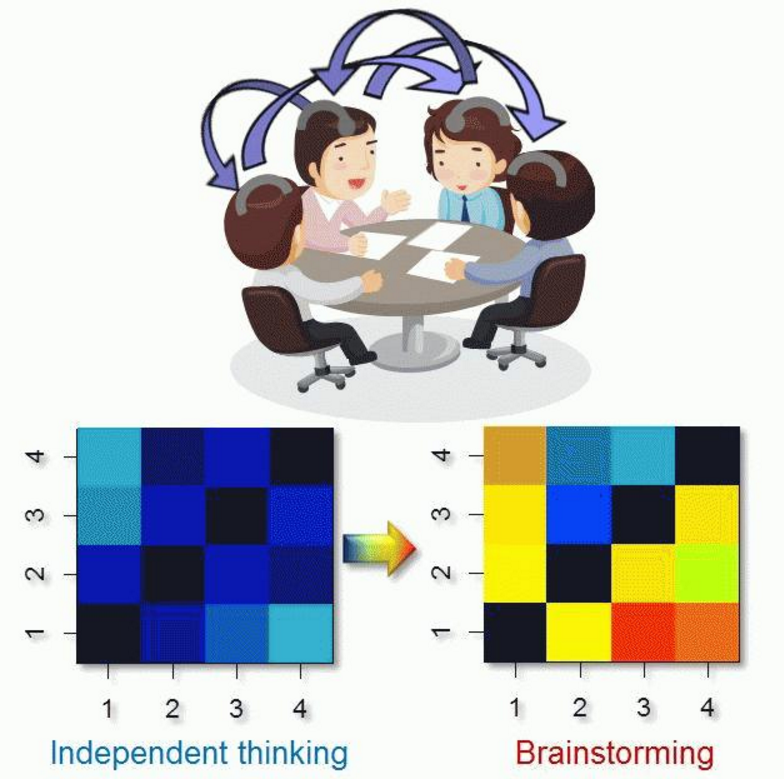
Furthermore, recent social neuroscience studies have departed from conventional cognition-brain mapping of an individual brain and they now combine the brain activities of two interacting people. In order to make further progress and extend this to broader social situations, we developed the Ultra-compact NIRS prototype which can simultaneously record and analyze the brain activities of up to 20 people. Using this system, we are conducting research on collective neural synchrony, which will lead to the development of technologies promoting communication and sympathy among people.
Examples:
[ms_icon icon=”fa-check” size=”16″ color=”#333333″ icon_box=”no” class=”” id=””] Decoding of fluctuating cognitive readiness from fMRI signals
When repeating a cognitive task, sometimes you will do well, and sometimes you will do poorly. We found that such change in performance can be predicted from the fluctuating activities of temporally coherent networks several seconds before the task. The decoding of cognitive readiness is applicable, but far from limited, to learning assistance and safe driving.
[ms_icon icon=”fa-check” size=”16″ color=”#333333″ icon_box=”no” class=”” id=””] Effect of communication on collective neural synchrony
Using the Ultra-compact NIRS system, we recorded prefrontal activities in groups of people while they were thinking about problems either together (brainstorming) or independently, and found that group-thinking enhanced collective neural synchrony. We are developing objective measures to evaluate quality of communication based on neural synchrony.
-
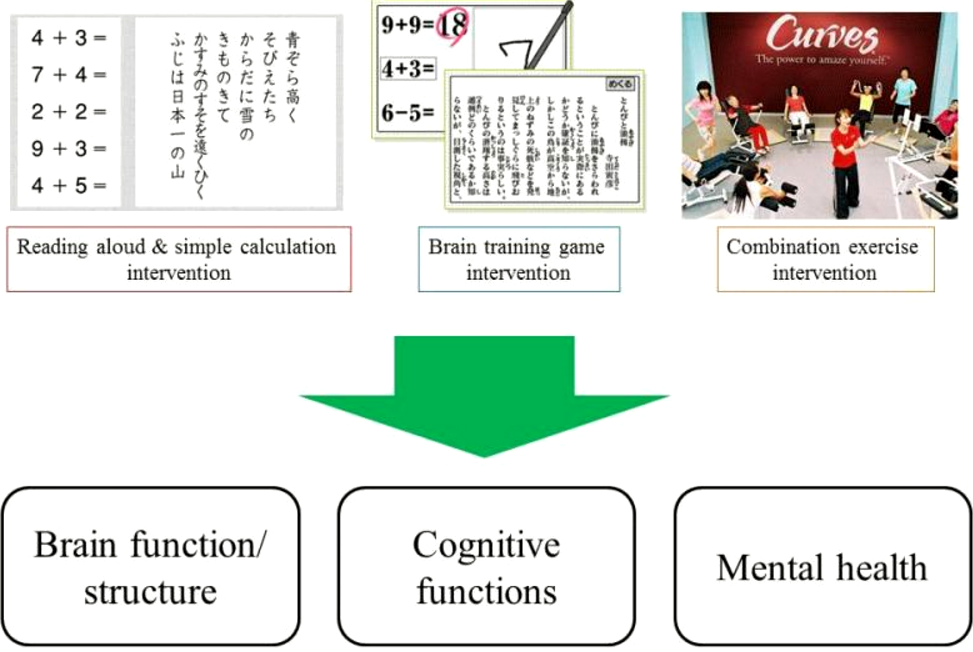
To improve cognitive functioning and mental health, we are developing new training methods (e.g., through video games, exercise, cognitive training) and also validating the scientific evidence of the new training methods.

Cognitive functions are complex mental processes which include memory, decision making, attention and processing speed. Unfortunately, cognitive functions decline with age. To cope with cognitive decline, we are developing a new method to improve cognitive functions and validating the evidence behind this new method.
The final goal of our smart ageing study is to disseminate new ways of dealing with cognitive decline and ways to develop and change our current social systems.
Examples:
[ms_icon icon=”fa-check” size=”16″ color=”#333333″ icon_box=”no” class=”” id=””] Is the brain training game we developed effective?
[ms_icon icon=”fa-check” size=”16″ color=”#333333″ icon_box=”no” class=”” id=””] Does daily activity lead to improvement in cognitive functions?
-
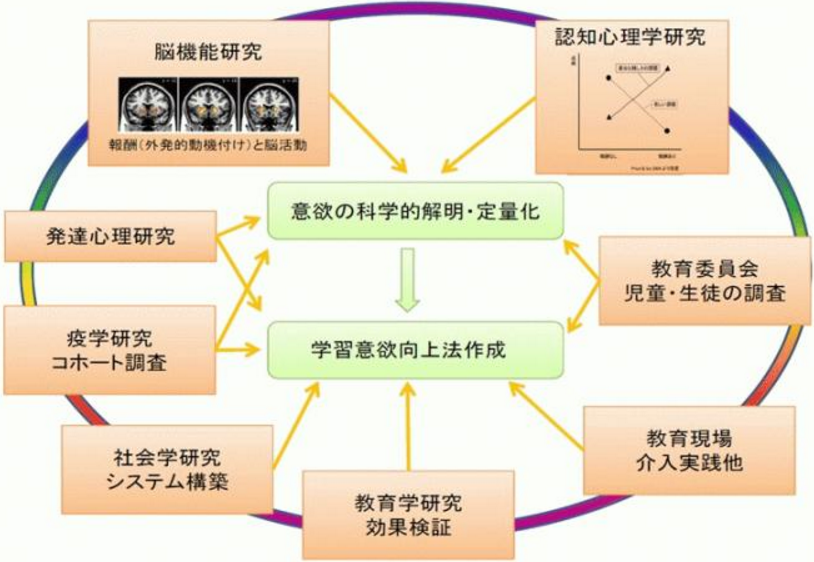
Using the new measures we’ve developed to quantitatively assess motivation for learning, as well as classroom observation and questionnaire surveys, we are engaged in research about student motivation to learn. As part of this investigation, we also look at the relationship between the motivation to learn and student lifestyles, family backgrounds, learning activities, attitudes in the classroom, and academic achievements.

Based on a partnership with the Sendai educational board, we are engaged in a research project on motivation for learning and its relationship with lifestyle, family background, learning activities, attitudes in the classroom, and academic achievement.
In particular we are focused on curiosity. What kinds of factors lie behind a high interest in learning and voluntary study? How does the relationship between curiosity and background factors change through each developmental stage? What is the key factor that maximizes an intervention’s effectiveness in inducing curiosity in a child?
Motivation for learning is also crucial for adults. It is essential for maintaining one’s intellectual level in adulthood and as we age. The factors underpinning adult curiosity are targets of our concern as well.
In addition, we are planning to introduce a part of the latest research outcomes in a booklet or a talk for children, parents, and teachers. In doing so, we hope to build a framework for parents, schools, and universities to cooperate with each other in enhancing children’s motivation for learning. Our ultimate goal is to establish a support system based on scientific evidence for children’s educational growth and development.
-
For sound development and growth of children.
The numerous activities of our group include developing a brain database for healthy children, clarifying normal brain development, elucidating lifestyle and genetic factors that influence brain development, and comparing the brains of normal patients using our brain database. With devices for scientific measurement, we also evaluate how products or systems which are developed by industries affect a consumer’s brain and we carry out collaborative research between industries and the university based on the results.”

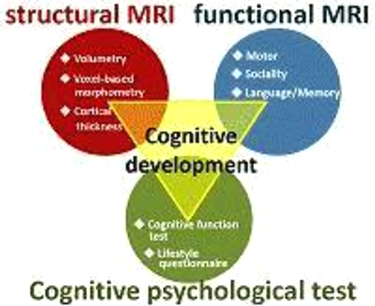
At present, few scientific data are available regarding the development of cognitive functions in healthy children. For the first three years of our study, we will therefore advance basic research using MRI to primarily investigate brain morphology, brain functions, and the development of cognitive functions in children. At the same time, we will examine which types of lifestyles and family member relationships will enrich a child’s spirit, using questionnaire surveys and cognitive function tests to determine the relationship between cognitive function development in children and the various environmental factors surrounding children today.
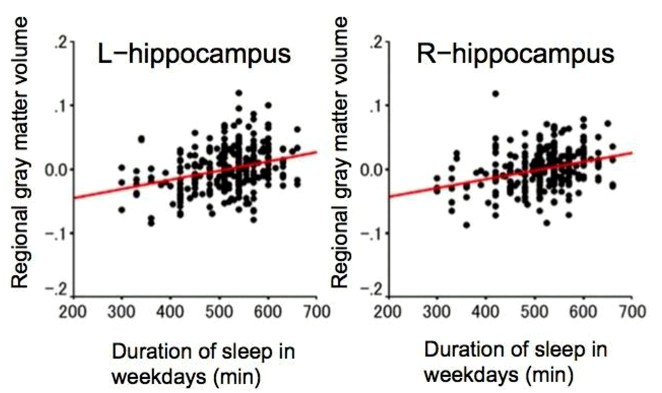
As we build a brain database of healthy children, we have been clarifying what is considered normal brain development in children [Ref. 1, 2, 3, 4]. We also identified the brain structures associated with children’s cognitive styles and abilities [Ref. 5, 6]. Furthermore, we’ve shown some evidence that lifestyle can influence a child’s brain structures. One example of this is the correlation between children’s sleep duration and gray matter volume in the hippocampus [Ref. 7, 8].
It is expected that the studies performed by our research division will help complete the basic theory for a new and practical teaching and learning system, which will facilitate children’s sound development and growth, as well as improve their appetite for learning, ability to think logically, their creativity, their intellectual curiosity, and their spirit of inquiry. It is also expected that our study will help clarify the mechanism of developmental disorders by comparing brains having a developmental disorder with the normal brains in our database.
Examples:
[ms_icon icon=”fa-check” size=”16″ color=”#333333″ icon_box=”no” class=”” id=””] Neuroscience-based proposal of a lifestyle preparing children’s brains for sound development
We propose a lifestyle based on brain science which will set up children’s brains for sound development. To do so, we begin by clarifying the correlation between a child’s lifestyle and changes after three years in their brain structures, brain functions, and cognitive abilities. The study will be conducted using our three-year longitudinal database which includes functional MRI, structural MRI, psychological tests, and lifestyle data.
[ms_icon icon=”fa-check” size=”16″ color=”#333333″ icon_box=”no” class=”” id=””] Development of a learning system based on age-appropriate brain development
Using functional MRI we are investigation the volume of age-appropriate gray matter and white matter in the brain. The volume appropriate for a given age is calculated using infant brain structural MRI and easy exercises, such as calculations and memorization. Other cognitive tests are also conducted to identify age-appropriate brain development. The goal of the study is to use these findings to help complete the basic theory for a new and specific teaching and learning system, which will improve children’s appetite for learning, their ability to think logically, their creativity, their intellectual curiosity, and their spirit of inquiry.